Our Pipeline
Pan-Amyloid Removal & Reversal Of The Underlying Pathology Of Systemic Amyloidosis
Making New Solutions Possible for Patients
AT ATTRALUS, WE SEE A CLEAR FUTURE WHERE MORE IS POSSIBLE FOR PATIENTS WITH SYSTEMIC AMYLOIDOSIS.
Using our proprietary pan-amyloid removal (PAR) technology, we are advancing a pipeline of novel therapeutics that remove toxic, disease-causing amyloid throughout the body with high specificity, and the potential to reverse disease pathology. We are pioneering first-in-class novel pan-amyloid therapies that bind to all types of amyloid, so that our therapies can be used for patients with all types of systemic amyloidosis and at all stages of disease.
Why It Matters
There is a critical unmet need for disease modifying therapies that can remove amyloid across all stages and types of systemic amyloidosis. The existing approved drugs for amyloidosis do not address the toxic amyloid deposits that are present and accumulate in organs and are the cause of disease burden, progressive organ failure and increased mortality in patients.
Our Pioneering Pan-Amyloid Pipeline

Our Pan-Amyloid Removal (PAR) Therapeutics
With the aim to treat all types of patients with systemic amyloidosis and open the door to reversing disease, at Attralus we are advancing our pipeline of product candidates for pan-amyloid removal. We have designed our novel biologics to achieve pan-amyloid removal with superior amyloid binding and immune-mediated phagocytosis.
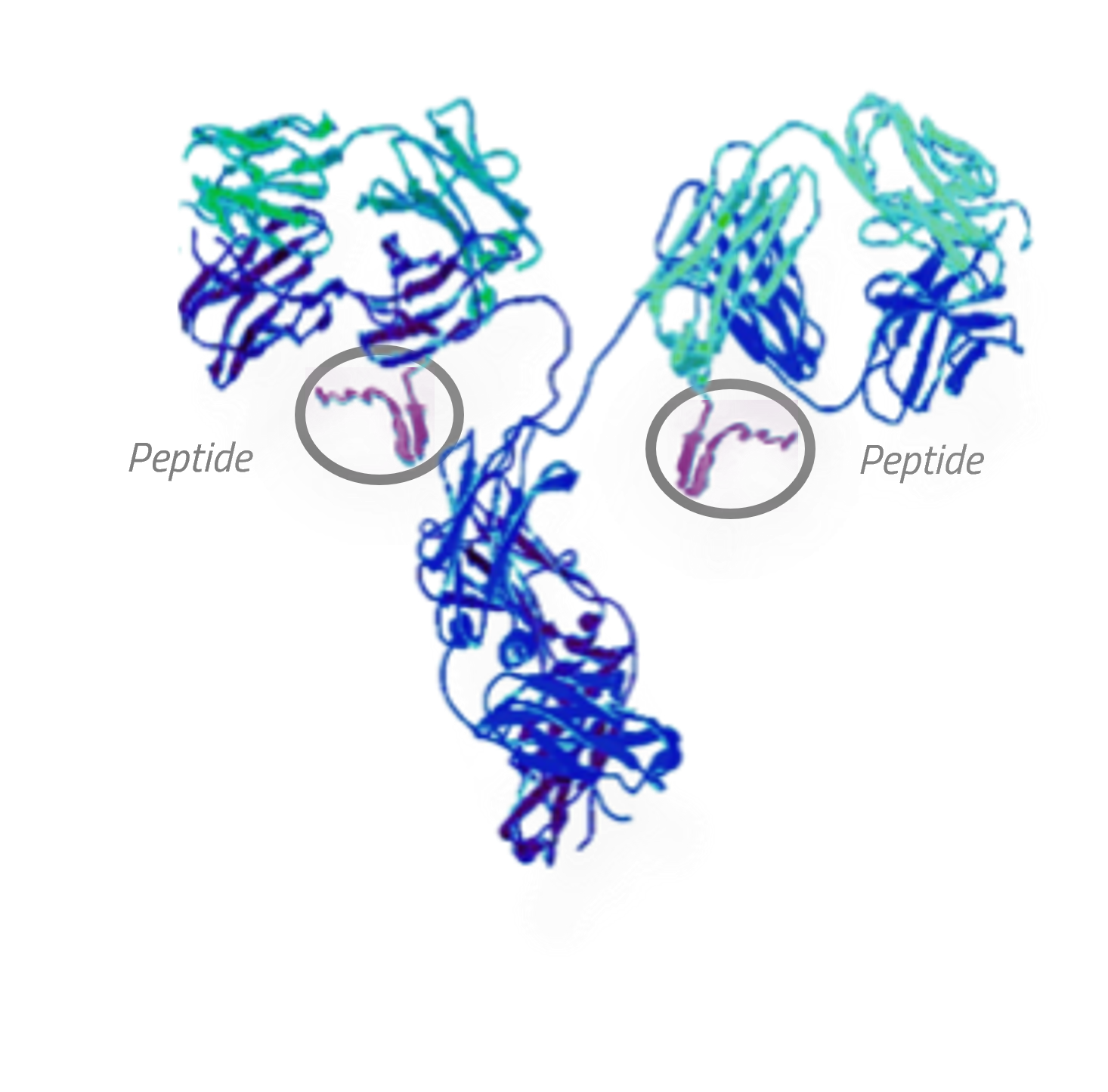
AT-02
PAR-Peptide + IgG1 Antibody
AT-02 is the company’s lead pan-amyloid removal (PAR) therapeutic candidate for systemic amyloidosis. AT-02 is a humanized IgG1 monoclonal antibody genetically fused with the company’s proprietary pan-amyloid binding peptide, enabling binding to multiple types of amyloid deposits. The Fc region of the antibody stimulates the immune system to remove amyloid deposits that are bound by AT-02. AT-02 uses a similar pan-amyloid peptide to 124I-evuzamitide, the company’s diagnostic agent, which has been shown in multiple clinical trials to selectively bind to amyloid deposits in the heart, liver, kidney, and other organs in multiple types of amyloidosis. As a result, the company expects AT-02 to bind specifically to amyloid in systemic amyloidosis patients. Preclinical data has shown the ability of AT-02 to bind to multiple amyloid types in major organs, induce macrophage mediated phagocytosis, and remove amyloid. AT-02 is currently being evaluated in a Phase 1 / 2 open label extension trial in AL amyloidosis patients.
Next Gen Candidates
PAR-Peptide + Antibody
Our Neurodegenerative Program
Attralus is developing the next generation of therapies for neurodegenerative diseases by simultaneously targeting multiple proteinopathies. Patients suffering from neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Lewy Body diseases, often have multiple proteinopathies. The company’s research is aiming to capitalize on this by binding to multiple misfolded protein aggregates including Aβ, Tau, and α-Synuclein. Attralus’ pan-amyloid product candidates provide an opportunity for therapeutics that can simultaneously target multiple proteinopathies in individual patients unlike approved therapies that target a single proteinopathy. The company is also exploring a blood brain barrier shuttle to potentially improve brain penetration.
Our Expanded Access Policy
Attralus understands the need and recognizes the importance of Expanded Access programs.

